CHEMISTRY THEORY and REVISION
Chemistry is a dynamic rapidly changing field.It is an extraordinary interesting subject.Chemistry is a connection to the universe.
Thursday, December 5, 2013
Saturday, February 25, 2012
CLICK THE VIDEO LINKS TO WATCH VIDEOS OF CHEMISTRY LESSONS.
General Chemistry....Contact for CHEMISTRY REVISION and THEORY GROUP CLASS(2014 / 2015)-+94774078055
 . "Payza"
. "Payza"
- Atomic structure
- Periodic table
- Bonding
Atomic structure
- Chemical Bonds
- Inter- molecular forces
Cathode Rays
Cathode ray is a beam of electrons that travel from negatively charged end to positively charged end of vacuumed tube,across a voltage difference between the electrodes placed at each end.The electrode at the negative end is called a cathode; the electrode at the positive end is called an anode
.
Properties of the cathode rays- Cathode rays come out at right angles to the surface of the cathode.
 VIDEO LINK
VIDEO LINK - Cathode rays move in straight lines.
- Cathode rays contain material particles having both mass and velocity.
 VIDEO LINK
VIDEO LINK - Cathode rays contain the smallest unit of negatively charged. VIDEO LINK
- Cathode rays produce X-ray when they strike a metal.
Mechanical properties of cathode rays video link
6 Cathode rays ionize a gas through which they pass
TESTING PROPERTIES OF CATHODE RAYS VIDEO LINK
Thomson 's model:-As a result of Thomson ' experiments,Thomson suggested that atoms consisted rings of negative electrons embedded in sphere of positive charge.The negative and positive charges balance and result in the being neutral.Thomson ' model for the atom was compared to a Christmas pudding,Plum pudding.
Plum Pudding Model Rutherford's atomic model
TESTING PROPERTIES OF CATHODE RAYS VIDEO LINK
Atomic Models
Plum Pudding Model Rutherford's atomic model

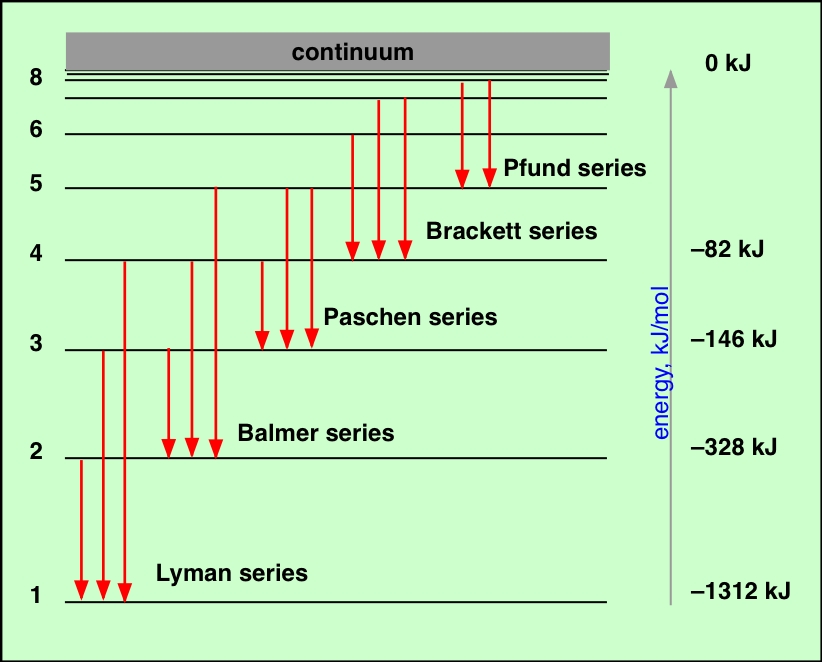
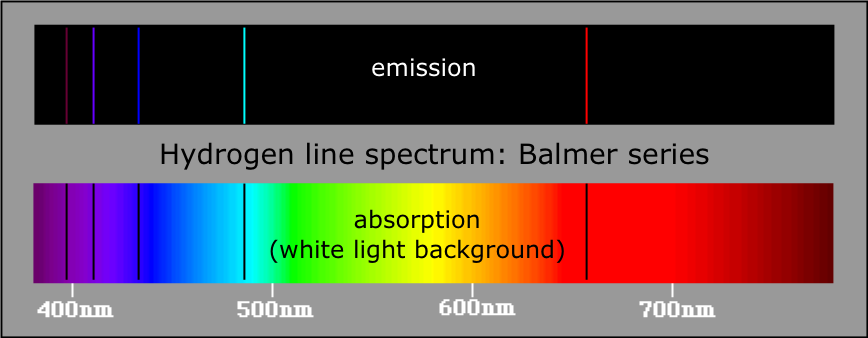
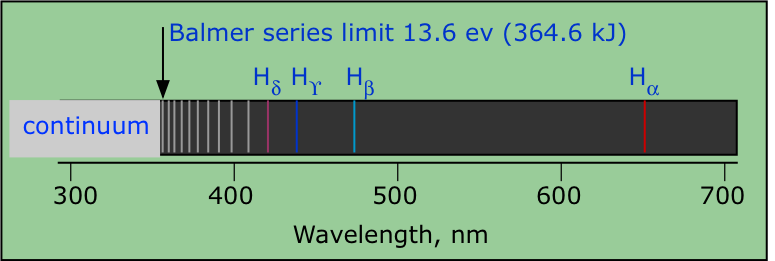
Atomic spectra
Studies on atomic spectra provided the evidence for the arrangement of electrons and their energies in atom. There are two types of atomic spectra which are atomic emission spectrum and atomic absorption spectrum. |

How Bhor model explain Hydrogen Spectrum
s-orbitals are spherical shape and p-orbitals are dumbbell shape.Each atomic orbital can hold either one or a maximum of two electrons.If the orbital contains two 'paired-up' electrons they will spinning in opposite directions.
s-orbital p-orbitals(dumbbell)
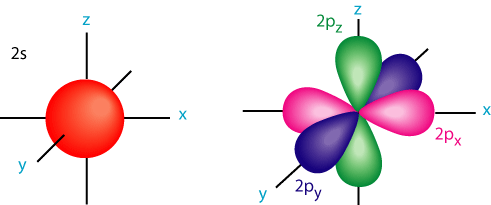
ELECTRON CONFIGURATION
The filling of atomic orbitals by electrons are governed by the three major guidelines.These are aufbau principle, Pauli's exclusion principle and Hund's rule.- Aufbau principle:-Electrons are filled in various orbitals in the increasing order of their energies.
- Pauli'exclusion principle:-No two electron in an atom can have all the four quantum numbers identical.According to this principle ,each orbital can accommodate at the most two electrons with opposite spins.
- Hund's rule:-This rule operates when the filling of orbitals are carried out.According to this rule,electrons enter one by one till all of them are singly occupied with parallel spins.

MASS SPECTROMETER
In 1919,Aston invented the mass spectrometer.This gave chemists a reliable and accurate method of comparing the relative atomic masses of atoms. There are five stages in mass spectrometer.
- Vaporisation:-the sample of element is vaporised.
- Ionisation:- positive ions are obtained from the vapour.
- Acceleration:- the positive ions are accelerated by an electric field.
- Deflection:-the positive ions are deflected by a magnetic field.
- Detection:- the ions are detected and record is made.
Periodic table
Ionization Energies of Elements
The ionization energy of an atom is strongly influenced by three atomic parameters.
Ionization Energies of Elements
The ionization energy of an atom is strongly influenced by three atomic parameters.
- The distance of the outermost electron from the nucleus. As this distance increases,the attraction of the positive nucleus for the negative electron will decrease.
- The size of the positive nuclear charge.As the nuclear charge gets more and more positive,its attraction for the outermost electron increases and consequently the ionization energy increases.
- The screening(shielding) effect of inner electrons.
Subscribe to:
Posts (Atom)


